Downstream of chemical-physical treatment plants, ozone is used as a solution for the elimination of hard-to-degrade organics including pigments and for the sterilization of water prior to discharge. Upstream of the chemical-physical treatments, ozone can chemically breakdown dissolved substances into materials that may be consumed by microorganisms.
Contact times vary from seconds to as much as a half-hour.
Ozonolysis is also used to treat wastewater treatment sludge, reducing the sludge volume and enhancing the production of biogas in downstream anaerobic digestion.
Ozone has found uses in treatment of pharmaceutical wastes and endocrine disruptors.
Because it is unstable and decays rapidly, ozone must be produced on site. Techniques of ozone manufacturing are UV-light and corona discharge. Corona ozone production is more common and more suited to large scale production than UV light.
UV radiation can be workable for manufacturing small quantities of ozone, for example in laboratories.
A corona ozone manufacturing unit includes an oxygen source, dirt filters, dryers, and electrical discharge elements. The ozone is produced as a direct result of the electric discharge. This electrical discharge breaks down the stable oxygen molecule and forms two oxygen radicals.
Ozone has a very short half-life, about 30 minutes in air, and degrades back into oxygen.
3O2 + Energy - 2 (O2 + O*) = 2O3
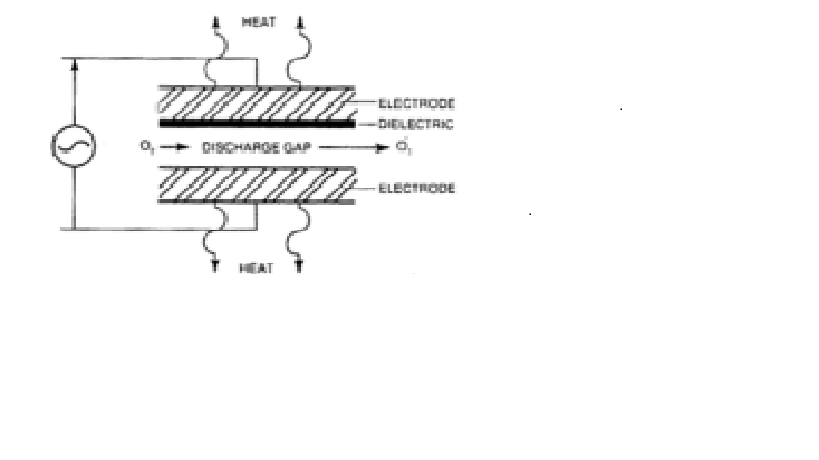
Figure 1: Ozone production by crown effect
The oxygen source is either atmospheric air (supplied by a compressor) or pure oxygen (which can be supplied by an oxygen generator, or sometimes in oxygen bottles) can be used. The incoming gas goes through molecular sieves and dust filters. Excessive heat from the electrodes is removed by cooling water, or by air (Figure 1).
Ozone treatment processes include units that destroy the ozone remaining after use. The units usually employ a magnesium oxide catalyst which accelerates the decomposition.
The production of ozone requires a lot of energy, and about 90% of the power supplied to the generator is wasted as light, sound, and heat. Important factors influencing ozone production are: oxygen concentration in the incoming gas, humidity and purity of the incoming gas, and cooling water temperature.
The presence of organic impurities in the incoming gas must be avoided, including impurities from leaks in the cooling units and leaks in the electrode cooling systems. The gas supplied to the generator must be very clean. Even when the hydrocarbon concentration in the oxygen is only 1 percent, ozone production approaches zero.
When the feed gas enters the ozone generator, it should be dry. Ambient air contains high humidity, which reacts with ozone. This leads to a reduction in the ozone yield per KWh. High humidity in the corona unit also leads to the production of nitrogen oxides. Nitric oxide can form nitric acid, which can cause corrosion.
In addition, hydroxide radicals are formed which combine with oxygen radicals and with ozone. All these reactions reduce the capacity of the ozone generator. At a dew point of -10 ° C, the capacity of the air powered generator is only 60% of the total achievable capacity. For oxygen-powered ozone generators, this capacity is higher, about 85%.
To prevent secondary reactions, the incoming air passes through a drying chamber which contains an aluminum compound comparable to a silicon gel. Ozone systems use two or more drying chambers in parallel. This allows regeneration to happen in one chamber while a different chamber is actively drying the air.
The production of ozone is accompanied by the generation of heat - the reaction is exothermic. This makes it important to cool the generator. The equilibrium shifts to the left at higher temperatures.
3 O2 = 2 O3
The ozone production rate decreases as the cooling water temperature increases. To limit the decomposition of ozone, the temperature in the drain hole should not exceed 25 °C. The general advice is that the cooling water temperature increase should be from 5 °C to 20 °C. It is important that the inlet air temperature is not too high.
This technology is now widespread, and you can choose ozone generators that produce from a few grams per hour to over 200 kg O3/hr. For the large units, power consumption is typically from 6.5 to 13 kWh depending on the temperature of the cooling water and the production concentration. The capital and operating costs of ozone generators have declined considerably in the past three decades, and the "ecological" nature of this technology, completely free of environmental impact, are the basis for the spread of the ozonation process. Ozone can be applied by itself or in combination with ultraviolet (UV) light, hydrogen peroxide (H2O2), and catalysts to create a higher performing Advanced Oxidation Process (AOP).
Ozone is one of the most effective disinfectants for single-cell organisms, lysing the cell membranes, acting in concentrations of less than 1 ppm and in a relatively short time. Ozone attacks cell membranes, damages them, and the cell dies. Common vegetative germs such as Staphylococcus Aureus are usually more sensitive than many other germs.
| Hydrophilic Virus | Reduction (%) | Minutes | Ozone (mg/L) |
| Poliovirus Type 1 | 75 - 99 | 10 | 0.2 |
| Virus enterici | 98 | 10 | 4.1 |
| Lipophilic virus | |||
| Rotavirus umano | 90 | 10 | 0.31 |
