The medical and industrial hygiene practices have developed "universal precautions" to limit occupational exposure to infectious diseases. Universal precautions were first developed in response to the AIDS crisis in the 1980’s for protecting healthcare workers from blood and body fluids visibly contaminated with blood. Universal precautions later evolved to include all body fluids and the new name for the practice was "standard precautions". However, most healthcare workers today use the terms “universal” and “standard” precautions interchangeably to mean the same thing – use of barrier devices and other methods to protect themselves from infectious diseases at work. The philosophy applies the precautionary principle and assumes all bodily fluids must be managed as if they contain HIV, HBV, and other bloodborne pathogens.
Universal precautions exist for one purpose: to stop or slow the spread of infection associated with medicine, and to protect healthcare workers from contact with patients' blood, body fluids, and secretions. They are the main form of infection prevention control in hospitals.
Barrier devices that protect healthcare workers from contact with infectious agents are called PPE – Personal Protective Equipment. PPE can be designed for one of two functions, either to protect the healthcare worker from contact with body fluids, or to protect the healthcare worker from inhaling infections such as tuberculosis or SARS.
OSHA requires that employers provide PPE when needed. Employees cannot be expected to provide their own PPE. Also, the employer must take charge of laundering and cleaning PPE as required and ultimately to dispose of it when the PPE has reached the end of its use. Most employers do not force employees to launder their own gowns. When regulated waste (blood products) are involved, OSHA actually stipulates that employers take care of gown cleaning.
It is advisable to have spill management kits where toxic drugs are routinely used and these kits can be effective for spot clean-up of medical waste spills, too. The spill kit has emergency one-size-fits-all PPE, a warning sign, and sometimes an absorbent. Some may include a brush and dust-pan and sealable plastic bags.
In facilities that treat patients, staff are advised to wash hands regularly. This includes staff who handle or transport waste. The standard procedure is to wash for 40 to 60 seconds with soap or detergent. Doctors and nurses performing surgery need to do a more thorough cleaning with alcohol, but for waste handling this is usually not required. The CDC has a guide for hand hygiene in healthcare settings, but the focus is more on treating patients than for managing waste.
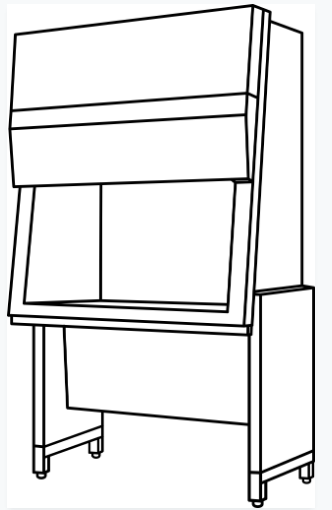
Like fume hoods employed for safety in chemical laboratories, biosafety cabinets find use in many clinical, diagnostic, and microbiological laboratories. Also called microbiological safety cabinets, they help keep workers safe primarily through channeling airflow away from the room and through HEPA filters. Lab personnel have a duty to keep the cabinets operating and to avoid disrupting airflow.
Biosafety cabinets are especially useful when people work with wet materials. Workers can decrease risk by avoiding splatter or doing anything that results in aerosol creation. Aerosols are particularly notorious as a vector of disease because they enter human lungs.
Either facility or regulatory personnel test cabinets by measuring air speed at the entrance.
There are different types of biosafety cabinets - different levels of safety promised to handle different levels of pathogen danger. As defined by the CDC, Class I cabinets must have an airflow rate of at least 75 ft/min into the cabinet. The exhaust from these cabinets can go into the building ductwork or back into the lab.
Class II cabinets are more often used in research laboratories as they are designed to avoid contamination of the microbiological samples inside the cabinet. As in Class I cabinets, exhaust air is filtered, but so is air entering the box. This protect the specimens under investigation from dust contamination.
Class III cabinets are glove boxes, similar to those used to process some radioactive materials. Materials that enter the box go through a double-door mechanism. The box is air tight. These cabinets are used in BSL-3 and BSL-4 work environments.
Some older BSCs have ultraviolet lamps installed to kill pathogens. This safeguard has fallen out of favor because of doubts as to its efficacy and to the fact that UV light can be bad for the skin of the workers. Further, mercuty vapor lamps were often used and a new awareness as to the toxicity of mercury and a phasing out of mercury equipment.
BSCs are periodically cleaned with disinfectant liquids and wipes. When a BSC is to be taken out of commission, it is cleaned with disinfecting vapor.
Technicians who work in biological safety cabinets preparing cytotoic medications typically wear surgical or procedure marks as well as gowns, goggles, and gloves.
Some labs include clean benches, which look like BSCs, but are in a way the opposite. They employ filtration for the air entering the container. The container air pressure is higher than the ambient room pressure. Air exits the cabinet to the lab. No worker protection is offered, but it does protect the material inside the cabinet from exposure to unfiltered air and hence most dust.
See also: worker safety.
